January/February 2018
Delivering New Perspectives on Cancer
BY EVA KAPLAN-LEISERSON
It’s difficult these days to find someone whose life hasn’t been touched by cancer. Approach any person walking down the street, and he or she could rattle off a number of relatives, friends, or coworkers who have struggled with the disease. Cancer is a great democratizer; it seems to come for us all in some form. If not us personally, someone we care about.
About 40% of US men and women will develop cancer in their lifetimes, according to 2017 data from the American Cancer Society.
Solving the cancer challenge necessitates an “all-hands-on-deck” approach, and engineers are among those contributing.
Innovative engineers and professional engineers in a wide range of engineering disciplines are lending their expertise to the fight. As the engineers on these pages explain, a multidisciplinary, collaborative approach is needed to stop cancer in its tracks.

Angela Belcher
Professor of Biological Engineering
and Materials Science and Engineering
Member, Koch Institute for Integrative
Cancer Research
MIT
“As an engineer, I’m a problem solver,” says Belcher. “I want to know what stands in the way of progress.” The MIT professor focuses her work in cancer on using science and technology to solve problems in order to drive advancements in diagnosis or treatment.
One of those problems is the difficulty in increasing survival rates for ovarian cancer. Symptoms for the disease are amorphous, and the cancer is usually discovered only at a late stage.
Belcher found that her work on nanomaterials could be used in new ways of spotting tumors. Her lab’s work includes genetically engineering bacterial viruses that can move materials. By engineering these bacterial phages to pick up materials such as carbon nanotubes that fluoresce, Belcher and her team have been able to create “probes” that bind to tumors that then appear to glow when viewed through an infrared camera.
The goal is to be able to find smaller tumors in less invasive ways. Experiments in mice have pinpointed tumors measuring just 0.2 millimeters. By partnering with Massachusetts General Hospital, the researchers are developing a system for real-time imaging that could help surgeons find and remove tiny tumors deep inside the body.
Ultimately, Belcher says, they’d like to develop a screening for high-risk patients that will find ovarian cancer earlier.
Belcher didn’t start out working on cancer. Her expertise in using biology to create better nanostructures had previously focused mainly on the areas of energy storage and electronic materials. But she was asked to add her lab to an integrated center at MIT for cancer research. The Koch Institute brings together faculty members in biology, a variety of engineering disciplines, and other areas for an interdisciplinary approach.
Belcher’s lab is now also working on ways to image, diagnose, and treat other cancers such as pancreatic cancer, melanoma, human glioma (brain tumors), and metastatic breast cancer.
“That’s a lot of cancers for a person who hasn’t been working on cancer for very long,” says the professor. “But I’m lucky to be in this building where I have so many people to learn from.” Not only do the faculty and students within the Koch Institute constantly collaborate with each other, but they also do so with physicians in the Harvard Medical School and medical schools around the country.
Engineering students get very excited about using their know-how to address problems in cancer, Belcher says. And the interdisciplinary collaborations make the work not only more interesting and exciting, but also enable a bigger impact.

Patrick Donahue, P.E.
CEO
Theragnostics Inc.
When Donahue was nine, his father died from a rare form of cancer. This personal history is part of what drives his work, he explains.
After earning a chemical engineering degree, the professional engineer became involved with the chemistry of radioactive materials while at DuPont. He has spent much of his career working in nuclear medicine and the development of radiopharmaceuticals. These radioactive materials are used in small amounts to diagnose or treat diseases such as cancer.
With diagnostic imaging such as a PET scan, a patient is injected with a radioactive tracer, which is attached to a targeting molecule that seeks out receptors on cancer cells. Detectors in a tunnel-like device can pick up the radiation leaving the body and produce a 3D image that helps determine cancer location and growth.
Theragnostics’ intellectual property is mainly centered around a radioactive tracer to detect prostate cancer. After the agent is injected into a patient who has been diagnosed, high-resolution imaging determines whether the cancer has spread. It’s a technique that’s more accurate and provides earlier results than other methods, Donahue says. The process can also be completed very rapidly to reduce the radiation dose.
In addition, the patient could be injected with a similar targeting molecule that has a therapeutic dose of radiation attached to it. Theragnostics is also working in this area, which Donahue calls “an exciting area for nuclear medicine.”
The company’s imaging agent has not yet been approved in the US, but Theragnostics has been performing clinical trials in Australia and the UK. Additional trials will start in the US in about a year.
Opportunities abound for engineers in nuclear medicine, Donahue notes, particularly with the extremely complex systems used for imaging.
“We’re learning that cancer is not one disease, but many diseases,” he says. Imaging methods such as the ones Theragnostics is working on are helping to drive a better understanding of those diseases.

Pierre Lane, P.Eng
Senior Scientist
British Columbia Cancer Research Center
Associate Professor of Professional Practice
Simon Fraser University
Associate Member,
Biomedical Engineering
University of British Columbia
The earlier cancer is detected, the better the chances to cure it or improve a patient’s outcome. Lane’s work focuses on using optical imaging to find cancer at stages where it’s most treatable. “That keeps me going,” he says, “knowing that [this work] will be applied to the greater good.”
Lane also didn’t start out concentrating in cancer. His optics work was previously in telecommunications, until the dot-com bust. A colleague suggested he apply his expertise to medicine.
Now, his research group is focusing on lung cancer, oral cancer, and gynecological cancer, which are difficult to access. They’re building small handheld devices that can be used with traditional tools such as endoscopes or bronchoscopes to detect cancer.
The devices take high-resolution pictures in the body without having to remove tissue from a patient. The end goal for such an “optical biopsy,” as Lane explains: a picture that could be analyzed for diagnosis on the spot.
Ideally, if the technology works out, biopsies wouldn’t have to be performed at all. “But that’s years down the road,” he says.
The work toward this goal isn’t new. But Lane says professional engineers can see problems from a different perspective. They can help generate unique solutions, he explains, or at least add new pieces to the puzzle.
PEs can also bring expertise to building devices and ensure safety standards are met in a clinical environment. “We can do things in a safe manner without infecting people with pathogens,” he says.
Lane’s advice to other professional engineers who may want to get involved in the medical arena: Listen to what physicians need. Instead of just applying a neat technology to a problem that doesn’t actually need to be solved, “make sure you’re solving useful problems.”

Thomas Milner
Professor of Biomedical Engineering
Cockrell School of Engineering
University of Texas at Austin
For cancer patients undergoing surgery, a major concern is that the surgeon has removed all of the cancer. Milner’s lab helped develop technology that could ensure that.
The MasSpec Pen is a handheld device that is applied to tissue to determine whether it’s cancerous.
When the pen contacts the tissue being sampled, it deposits a droplet of water. Small molecules move onto the droplet, then the water is brought back into the instrument, ionized, and analyzed. The device sorts and classifies molecules with mass spectrometry, using an algorithm that examines their particular signature.
Surgeons get better information about which tissue to cut or preserve, improving treatment and reducing the chances that cancer will reoccur.
The MasSpec Pen has been validated on a number of different types of cancer. It was the vision of chemistry professor Livia Schiavinato Eberlin, who enlisted the help of Milner’s lab to engineer the device.
The current method of determining cancer’s boundaries, frozen section analysis, requires 30 minutes or more to prepare samples and have them examined by a pathologist. This can increase risks of infection and the negative effects of anesthesia, and in some types of cancer, generate unreliable results in up to 20% of cases.
By contrast, the MasSpec Pen works in about 10 seconds and was more than 96% accurate in tests on tissues removed from cancer patients.
The team plans to start clinical testing the device in surgeries in 2018.
“Human health is one of the last frontiers,” explains Milner. As people are living longer and increasing their standard of living, he says, engineers are a critical component to developing solutions. He sees a growing trend of engineering and science departments getting involved with medical research and applications.
“What needs to be realized and how it needs to work often come from people who may not be engineers,” he says. “But the constraints on the design of the device, what it needs to do, engineers are the people who actually make it realized. They’re an essential component in designing and constructing these devices.”

Sunitha Nagrath
Associate Professor of
Chemical Engineering
College of Engineering
University of Michigan
Nagrath wants to change the way cancer is diagnosed. Early diagnosis, she says, is the Holy Grail. “Everybody speaks about it; it’s really hard to achieve.”
Her research aims to replace the tissue biopsy with a “liquid biopsy” using blood. This could enable more frequent monitoring and customized treatment of patients, as well as screening for high-risk individuals.
Nagrath has long been interested in fluid mechanics. In her postdoc at Harvard Medical School, she learned about rare circulating tumor cells in the blood of cancer patients. It started her thinking about whether blood could be analyzed for the presence of these cells and how fluid mechanics principles could be applied for cell separation.
Engineers could offer better and less invasive technology, she thought, to examine tumors through the blood.
Nagrath has led the development of a “labyrinth chip” that sends blood samples through a maze of channels thinner than a human hair. This enables the separation of cancer cells for analysis and is “label-free” technology, not requiring the use of antibodies that prespecify cell type.
The channels in the business card-sized chip enable capture of both large and small cells more precisely and in high volumes. The chip can typically process 2.5 milliliters of blood per minute.
Nagrath’s lab comprises chemical, electrical, and biomedical engineers. And as part of the Biointerfaces Institute, she’s involved in interdisciplinary efforts with 25 research groups from the University of Michigan’s schools of engineering, dentistry, medicine, and pharmacy. This approach is essential to success, she says.
Having clinicians’ input from Day Zero “really changes the mindset in our students and guides the design principles,” she says. The collaboration is also key for developing solutions faster.
It’s important for engineers to team up with clinicians, she says, because the latter may not know what engineers can do. “When I first started interacting with clinicians, some of the problems they described, I said, ‘Wow, we can do this better. Why are we doing it that way?’”
Often the status quo is maintained because people don’t know a better way, she continues. “But as engineers, that’s what we do. We innovate, create, build, and develop.”
The time is right for engineers to actively look for problems they can solve in cancer, Nagrath believes. She emphasizes the need to be bold and push past boundaries. “Don’t limit yourself by the traditional definitions. Engage beyond what you think you can do.”


 Volunteering at NSPE is a great opportunity to grow your professional network and connect with other leaders in the field.
Volunteering at NSPE is a great opportunity to grow your professional network and connect with other leaders in the field. The National Society of Professional Engineers (NSPE) encourages you to explore the resources to cast your vote on election day:
The National Society of Professional Engineers (NSPE) encourages you to explore the resources to cast your vote on election day: THE WORK IN BELCHER’S LAB FOCUSES ON UNDERSTANDING AND HARNESSING NATURE’S PROCESSES TO DESIGN MATERIALS AND DEVICES FOR ENERGY, THE ENVIRONMENT, AND MEDICINE.
THE WORK IN BELCHER’S LAB FOCUSES ON UNDERSTANDING AND HARNESSING NATURE’S PROCESSES TO DESIGN MATERIALS AND DEVICES FOR ENERGY, THE ENVIRONMENT, AND MEDICINE.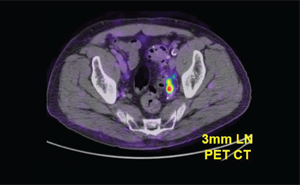 A SMALL TUMOR DETECTED BY THE THE RAGNOSTICS PROSTATE CANCER AGENT IN A CLINICAL TRIAL
A SMALL TUMOR DETECTED BY THE THE RAGNOSTICS PROSTATE CANCER AGENT IN A CLINICAL TRIAL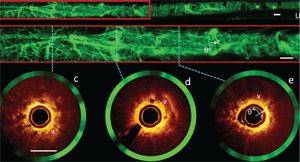 IMAGES FROM A HUMAN AIRWAY ACQUIRED BY A FIBER-OPTIC IMAGING CATHETER AND SYSTEM DEVELOPED IN LANE’S LAB. THE TWO OPTICAL IMAGING MODALITIES USED HAVE EACH SHOWN PROMISE FOR THE EARLY DETECTION OF LUNG CANCER.
IMAGES FROM A HUMAN AIRWAY ACQUIRED BY A FIBER-OPTIC IMAGING CATHETER AND SYSTEM DEVELOPED IN LANE’S LAB. THE TWO OPTICAL IMAGING MODALITIES USED HAVE EACH SHOWN PROMISE FOR THE EARLY DETECTION OF LUNG CANCER.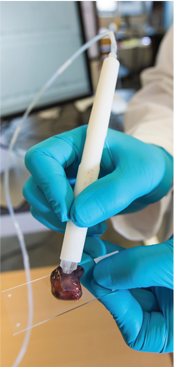 DEMONSTRATION OF THE ENGINEERED PEN ON A HUMAN TISSUE SAMPLE
DEMONSTRATION OF THE ENGINEERED PEN ON A HUMAN TISSUE SAMPLE THE DESIGN OF THE LABYRINTH CHIP ENABLES CANCER CELLS TO BE SEPARATED OUT WHILE REDUCING CONTAMINATION BY OTHER TYPES OF CELLS. CREDIT: ERIC LIN
THE DESIGN OF THE LABYRINTH CHIP ENABLES CANCER CELLS TO BE SEPARATED OUT WHILE REDUCING CONTAMINATION BY OTHER TYPES OF CELLS. CREDIT: ERIC LIN